A Carbon Bond in Ethane Ch3ch3 Is Best Described a
Organic molecules contain at least A three carbon to hydrogen bonds. Sp3Hybridization in Ethane CH 3CH3 Geometry of Ethane CH3CH3 All bond angles 1095 So ethane is tetrahedral at both carbons.

Solved 3 A Carbon Hydrogen Bond In Ethane Ch3ch3 Is Best Chegg Com
It contains a pi.
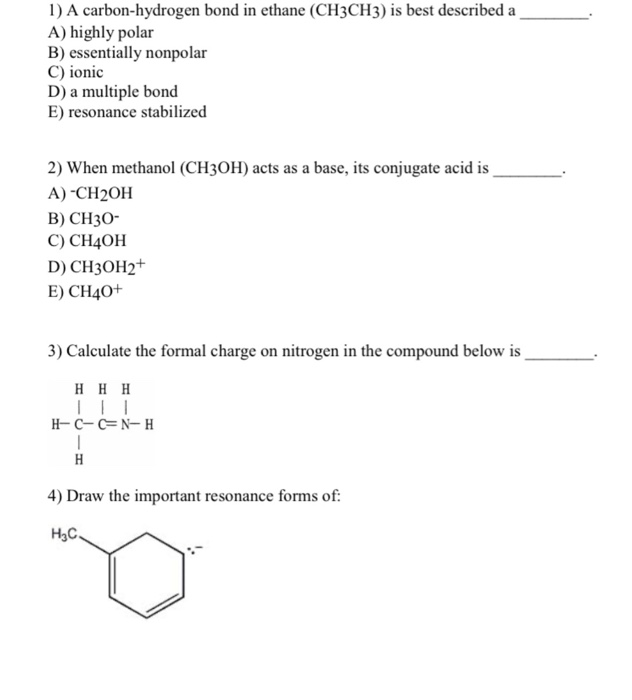
. CC H H H H H H From ChemBio3D free to UofM. Choose the one alternative that best completes the statement or answers the question. What type of chemical bonding is present in the following compound.
Ethane is a gas and therefore volatilization from soil and water is expected to be the most important fate process. 3 A carbon-hydrogen bond in ethane CH3CH3 is best described A highly polar B essentially nonpolar C ionic D a multiple bond. CH3CH3 ETHANE is a covalent bond.
11 Due to electron delocalization one would predict that the carbon-oxygen bond in acetamide CH3CONH2 _____. Carbon changes oxidation state. 3 A carbon-hydrogen bond in ethane CH3CH3 is best described A highly polar B essentially nonpolar C.
Rank the following in order of increasing boiling. The simplest molecule with a carbon-carbon bond is ethane C 2 H 6. No there are no hydrogen bonds in CH3-CH3 ethane.
What is chemical bond ionic bond covalent bond. A carbon-hydrogen bond in ethaneCH3Ch3 is best described as _____. A carbon-hydrogen bond in ethane CH3CH3 is best described a _.
Chemistry questions and answers. A carbon-hydrogen bond in ethane CH3CH3 is best described a ________. A carbon-hydrogen bond in ethane is best described as.
Multiple choice questions 1. In this bondwhich of the following best describes the charge on the carbon atom. A highly polar B.
B has more double bond character than the carbon-oxygen. In the ethane molecule the bonding picture according to valence orbital theory is very similar to that of methane. Both carbons are sp 3 3 orbital from each of the carbons.
A carbon-hydrogen bond in ethane CH3CH3 is best described as essentially nonpolar. Asked Aug 28 2020 in Chemistry by Paramedic. Acetylene is reduced to ethane.
In a carbon atom the 2s and 2p orbitals are equal in energy. A chemical bond is a lasting attraction between atoms ions or. FALSE 11 A carbon-hydrogen bond in ethane CH3CH3 is best described a _____.
In ethane CH 3 CH 3 both carbons are sp 3 -hybridized meaning that both have four bonds with. A carbon-hydrogen bond in ethane CH3CH3 is best described as _____. View Notes - Multiple questions from CHEM 237 at Illinois Institute Of Technology.
Asked Aug 28 2020 in Chemistry by Paramedic. 7A carbon-hydrogen bond in ethane CH3CH3 is best described a _____. Both carbons are sp 3-hybridized meaning that both have.
A carbon-hydrogen bond in ethane CH3CH3 is best described a _____. A Carbon-hydrogen Bond in Ethane Ch3ch3 Is Best Described a Get link. CH 3 CH 2 CH 2 CH 2 Br.
The Henrys Law constant for ethane is estimated as 05 atm-cu. Other Apps - April 11 2022 Snyder Jon Antilla. Option B is correct View answer additonal benefits from the subscription Subscribe.
A multiple bond E. A multiple bond e. In the ethane molecule the bonding picture according to valence orbital theory is very similar to that of methane.
This is because carbon and hydrogen have similar electronegativities. Essentially nonpolar equality.

Solved 1 A Carbon Hydrogen Bond In Ethane Ch3ch3 Is Best Chegg Com
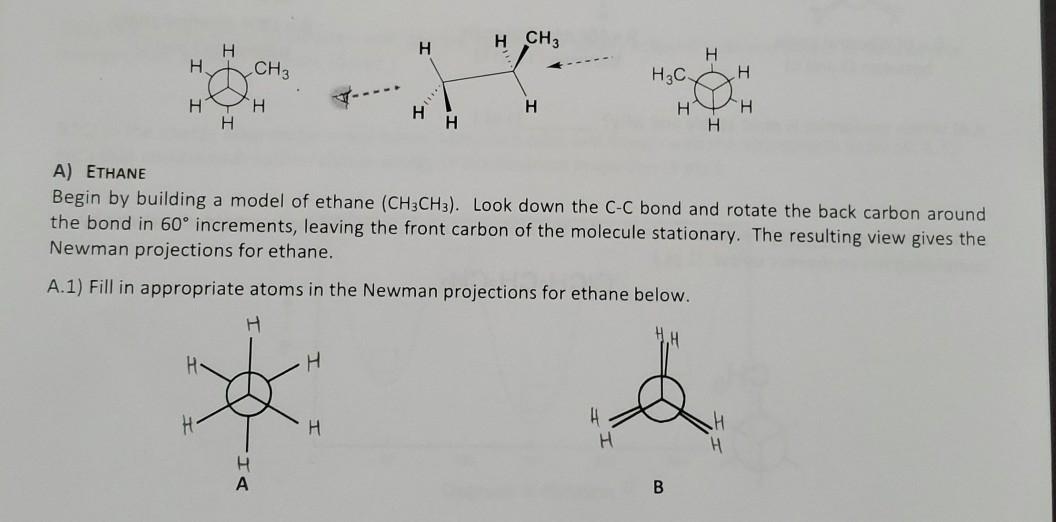
Solved H Ch3 H H H Ch3 H3c H H H H N H H H H H H A Ethane Chegg Com
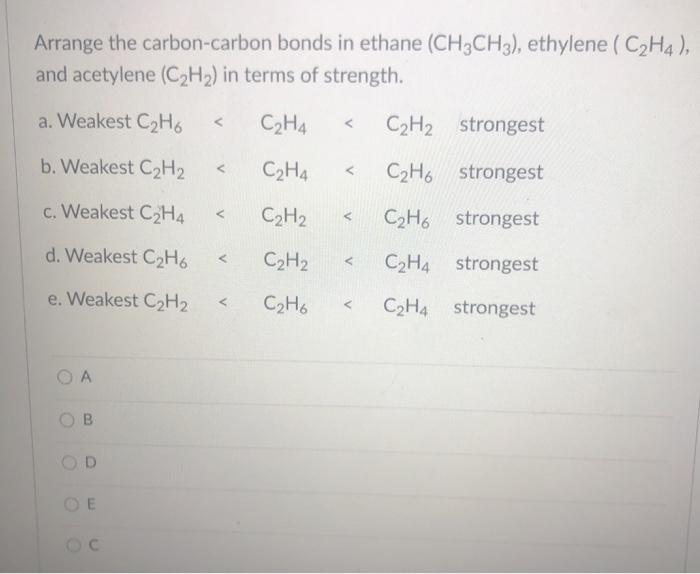
Solved Arrange The Carbon Carbon Bonds In Ethane Ch3ch3 Chegg Com

Solved A Carbon Carbon Bond In Ethane Ch3ch3 Is Best Chegg Com
Comments
Post a Comment